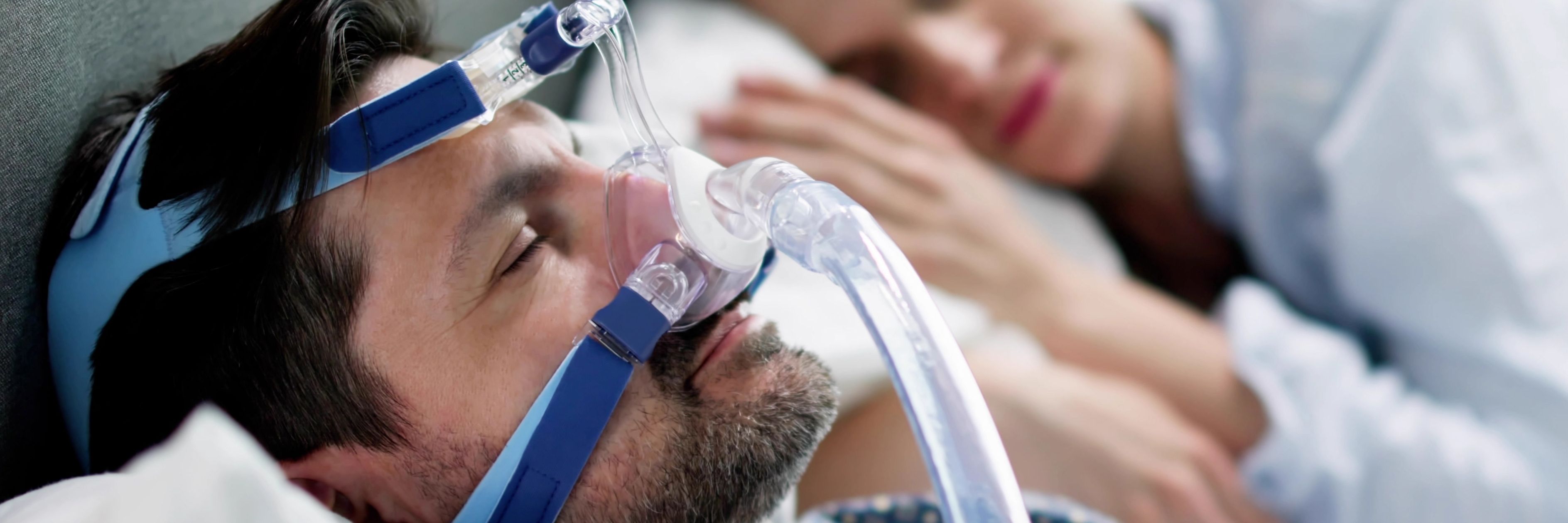
Sleep apnoea breathing therapy depends not only on the performance of the therapy device itself, but also on the safety and reliability of the interface that connects the patient to it. EN ISO 17510:2025 addresses this critical link by specifying safety and performance requirements for masks and application accessories used in sleep apnoea therapy.
EN ISO 17510:2025 specifies the safety and performance requirements for masks and accessories used to connect a patient to sleep apnoea breathing therapy equipment. While the therapy equipment itself (the blower/machine) is covered by a separate standard (EN ISO 80601-2-70), this document focuses strictly on the interface – the mask, headgear, and connecting elements – that delivers the air to the patient.
It covers critical aspects, such as:
- Biocompatibility: Ensuring materials touching the skin or gas pathways are safe.
- Rebreathing Protection: Preventing the inhalation of exhaled Carbon Dioxide (CO2).
- Acoustic Energy: Limiting and measuring the noise produced by the mask to ensure sleep quality.
- Information Supply: Mandating what instructions and warnings manufacturers must provide to users.
Updates and changes to the standard
This second edition (2025) cancels and replaces the first edition from 2015. It introduces several vital updates to reflect technological advancements and safety data, including:
- Magnet Disclosure: New requirements were added for masks and headgear that utilize magnets, including specific warnings for patients with implants (like pacemakers).
- Biocompatibility Updates: The standard now references EN ISO 18562-1 for the evaluation of gas pathways, addressing risks of substances leaching into the air stream.
- Harmonization: The text has been harmonized with EN ISO 20417 for information supplied by manufacturers and updated regarding cleaning and disinfection processing requirements.
- Noise Requirements: Methods for determining sound power and pressure levels have been updated.
Why does this standard matter?
Sleep apnoea therapy relies on a sealed system to deliver therapeutic pressure. The mask is the most variable part of this system: if it leaks, is uncomfortable, or retains CO2, the therapy fails.
This standard is crucial because:
- It prevents asphyxiation: It mandates anti-asphyxia valves for masks covering the mouth, ensuring patients can breathe fresh air even if the machine loses power.
- It protects vulnerable users: The new inclusion of magnet warnings addresses a life-threatening risk for patients (or their bed partners) who have metallic implants or medical devices like cerebrospinal fluid (CSF) shunts or aneurysm clips.
- It ensures material safety: By strictly applying EN ISO 10993 and EN ISO 18562, it ensures that materials in long-term contact with the skin or lungs do not cause toxic reactions.
Benefits for industry and society
The standard offers many benefits for patients and society as a whole:
- Safety Assurance: Patients can trust that compliant masks have been rigorously tested for CO2 flushing (rebreathing) and biocompatibility.
- Better Sleep Hygiene: By standardizing how acoustic energy (noise) is measured and declared, patients and bed partners are protected from excessive disturbance, which is critical for adherence to therapy.
- Clearer Instructions: The standard mandates clear user instructions regarding cleaning cycles and the 'shelf life' of the device, preventing the use of degraded equipment.
The document also brings benefits for industry:
- Regulatory Clarity: It aligns with International Medical Device Regulators Forum (IMDRF) essential principles, helping manufacturers meet global regulatory requirements.
- Standardized Testing: It provides normative annexes (Annex B through G) with precise test setups for exhaust flow, pressure drop, and noise, creating a level playing field for product verification.
Practical applications of the standard
Engineers, manufacturers, and testing laboratories use this standard for:
- Design Validation: Verify that the pressure drop across the mask does not exceed 10 hPa at 50 l/min during a single fault condition (e.g. machine failure).
- Labelling Creation: Draft user manuals that explicitly warn against using magnetic masks near devices like cardioverter defibrillators.
- Material Selection: Choose polymers and silicones that pass the rigorous long-term exposure biocompatibility tests required for devices used for more than 30 days.
- Noise Measurement: Utilize specific microphone positions and reflecting planes to calculate the A-weighted sound power level for product specification sheets.
Consider a patient undergoing CPAP therapy who also has an implanted pacemaker. In the past, headgear using convenient magnetic clips might have posed a silent risk of interfering with the pacemaker's function.
Under EN ISO 17510:2025, the manufacturer is now required to:
- Measure the magnetic flux density of the clips.
- Provide a specific warning in the user manual, stating: "WARNING: keep the headgear and their magnets away from devices and implants that can be affected by magnetic fields".
- Explicitly list contraindicated implants, such as insulin pumps, cochlear implants, and stents.
This specific update directly prevents potentially fatal interactions in the home environment, ensuring that the convenience of a mask does not compromise the user's cardiac safety.
EN ISO 17510:2025 reinforces the essential role of masks and accessories in sleep apnoea therapy, translating technical requirements into tangible safety and usability benefits for patients. By incorporating updated biocompatibility approaches, clearer information requirements, refined noise measurement methods, and new safeguards for magnetic components, the standard responds to evolving technologies and emerging risks. Ultimately, it supports safer home therapy, greater patient confidence, and regulatory clarity for manufacturers, ensuring that comfort and convenience never come at the expense of patient safety.
Special thanks go to the experts in CEN/TC 215 ‘Respiratory and anaesthetic equipment’, and to BSI, who hold the Secretariat of the TC, for their work on the standard.
Jennifer OGBONNA
jogbonna@cencenelec.eu



